Electron coupled FeS2/MoS2 heterostructure for efficient electrocatalytic ammonia synthesis under ambient conditions†
Abstract
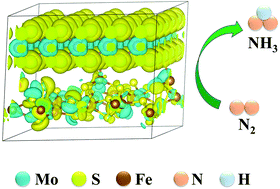
Developing efficient ammonia synthesis technology under ambient conditions is of vital importance. In this work, an FeS2 coupled MoS2 heterostructure with ultrathin features was designed by a one-step hydrothermal process for the electrochemical nitrogen reduction reaction. Density functional theory calculations reveal that the electronic structure of MoS2 greatly changes with the introduction of FeS2. The modulated electronic structure of MoS2 not only exhibits enhanced conductivity but also facilitates the activation of N2 molecules due to its abundant electronic region. The optimized FeS2/MoS2 nanosheet heterostructure achieves a high NH3 yield rate of 2.59 μmol h−1 mg−1 and a FE of 4.63% at −0.3 V vs. RHE. Besides, the well-designed nanocomposite also shows excellent selectivity without N2H4 by-products and exhibits good stability after electrocatalysis for 48 hours.


 Please wait while we load your content...
Please wait while we load your content...