Abstract
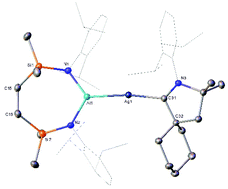
Reactions of the seven-membered heterocyclic potassium diamidoalumanyl, [K{Al(SiNDipp)}]2 (SiNDipp = {CH2SiMe2NDipp}2; Dipp = 2,6-di-isopropylphenyl), with a variety of Cu(I), Ag(I) and Au(I) chloride N-heterocyclic carbene (NHC) adducts are described. The resultant group 11-Al bonded derivatives have been characterised in solution by NMR spectroscopy and, in the case of [{SiNDipp}Al–Au(NHCiPr)] (NHCiPr = N,N′-di-isopropyl-4,5-dimethyl-2-ylidene), by single crystal X-ray diffraction. Although similar reactions of LAgCl and LAuCl, where L is a more basic cyclic alkyl amino carbene (CAAC), generally resulted in reduction of the group 11 cations to the base metals, X-ray analysis of [(CyCAAC)AgAl(SiNDipp)] (CyCAAC = 2-[2,6-bis(1-methylethyl)phenyl]-3,3-dimethyl-2-azaspiro[4.5]dec-1-ylidene) provides the first solid-state authentication of an Ag–Al σ bond. The reactivity of the NHC-supported Cu, Ag and Au alumanyl derivatives was assayed with the isoelectronic unsaturated small molecules, N,N′-di-isopropylcarbodiimide and CO2. While these reactions generally provided products consistent with nucleophilic attack of the group 11 atom at the electrophilic heteroallene carbon centre, treatment of the NHC-supported copper and silver alumanyls with N,N′-di-isopropylcarbodiimide yielded less symmetric Cu–C and Ag–C-bonded isomers. In contrast to the previously described copper and silver alumanyl derivatives, [(NON)Al(O2C)M(Pt-Bu3)] (M = Cu or Ag; NON = 4,5-bis(2,6-di-isopropylanilido)-2,7-di-tert-butyl-9,9-dimethylxanthene), which were prone to facile CO extrusion and formation of carbonate derivatives, the NHC-supported dioxocarbene species, [(NHCiPr)M(CO2)Al(SiNDipp)] (M = Cu, Ag, Au), are all stable at room and moderately elevated temperatures. The stabilising role of the NHC co-ligand was, thus, assessed by preparation of the t-Bu3P adducted copper-alumanyl, [(t-Bu3P)CuAl(SiNDipp)]. Treatment of this latter compound, which was also structurally characterised by X-ray analysis, with both N,N′-di-isopropylcarbodiimide and CO2 again provided smooth heteroallene insertion and formation of the relevant Cu–C-bonded products. Although both compounds were quite stable at room temperature, heating of [(t-Bu3P)Cu(CO2)Al(SiNDipp)] at 60 °C induced elimination of CO and formation of the analogous carbonate, [(t-Bu3P)Cu(OCO2)Al(SiNDipp)], which was identified by 13C and 31P NMR spectroscopy. Reflective of the more reliable nucleophilic behaviour of the gold centres in these group 11 alumanyls, computational (QTAIM and NBO) analysis highlighted a lower level of covalency of the Al–Au linkage in comparison to the analogous Al–Cu and Al–Ag interactions. Although substitution of the co-ligand significantly perturbs the charge distribution across the Cu–Al bond of [LCuAl(SiNDipp)] (L = NHCiPr or t-Bu3P), only a negligible difference is observed between the phosphine-coordinated copper systems derived from either the [SiNDipp]- or (NON)-based alumanyl ligands. Computational mapping of the reaction profiles arising from treatment of the various group 11 alumanyls with N,N′-di-isopropylcarbodiimide indicates that the observed formation of the Cu–N and Ag–N bound isomers do not provide the thermodynamic reaction outcome. In contrast, examination of the CO2-derived reactions, and their potential toward CO extrusion and subsequent carbonate formation, implies that the identity of the co-ligand exerts a greater influence on this aspect of reactivity than the architecture of the diamidoalumanyl anion.


 Please wait while we load your content...
Please wait while we load your content...