Host–guest complexes vs. supramolecular polymers in chalcogen bonding receptors: an experimental and theoretical study†
Abstract
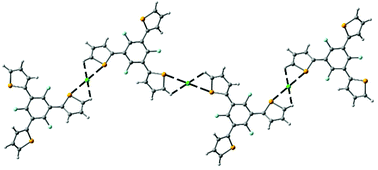
We describe here a comparative study between two tripodal anion receptors based on selenophene as the binding motif. The receptors use benzene or perfluorobenzene as a spacer. The presence of the electron-withdrawing ring activates the selenium atom for anion recognition inducing the formation of self-assembled supramolecular structures in the presence of chloride or bromide anions, which are bonded by the cooperative action of hydrogen and chalcogen bonding interactions. DOSY NMR and DLS experiments provided evidence for the formation of the supramolecular structures only in the presence of a perfluorobenzene based anion receptor while the analogous benzene one shows the classical anion/receptor complex without the participation of the selenium atom. The energetic and geometric features of the complexes of both receptors with the Cl and Br anions have been studied in solution. These results combined with the molecular electrostatic potential (MEP) surface plots allow us to rationalize the quite different behaviors of both receptors observed experimentally.


 Please wait while we load your content...
Please wait while we load your content...