A heteroditopic NHC and phosphine ligand supported ruthenium(ii)-complex: an effective catalyst for the N-alkylation of amides using alcohols†
Abstract
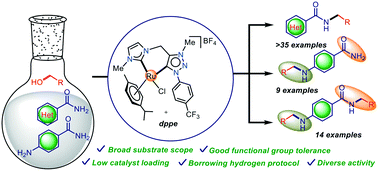
A ruthenium(II) complex of a chelated heteroditopic N-heterocyclic carbene ligand in combination with a phosphine ligand was uncovered to be a highly effective catalyst for the N-alkylation of diverse amides using readily available primary alcohols. A wide range of secondary amides was thus obtained in excellent yields (up to 98%) employing a low catalyst (2c) loading of 0.2 mol% and a substoichiometric amount of base. The 1H NMR and ESI-MS analyses support the participation of a N-heterocyclic carbene and phosphine supported Ru–H species in the catalytic cycle and the mechanistic studies including the deuterium labelling experiment suggest the involvement of a borrowing hydrogen protocol. Additionally, the present catalytic system was also revealed to be efficient for the selective mono-alkylation and unsymmetrical di-alkylation of 4-aminobenzamides which have not been studied before to the extent of our knowledge.


 Please wait while we load your content...
Please wait while we load your content...