Photothermal synergy for efficient dry reforming of CH4 by an Ag/AgBr/CsPbBr3 composite†
Abstract
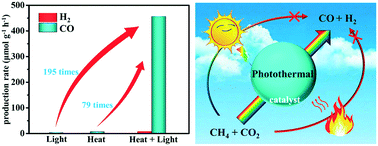
Dry reforming of CH4 by photothermal catalysis is considered to be a promising approach to produce syngas. It can not only store useful chemical energy, but also consume CO2 and CH4 to mitigate the greenhouse effect. Herein, we anchor Ag/AgBr on CsPbBr3 through an in situ ion exchange route to construct a composite material, which exhibits an excellent photothermal synergistic effect in the dry reforming of CH4. The CO production rate of the 7 wt% Ag/AgBr/CsPbBr3 composite through photothermal catalysis is 456.15 μmol g−1 h−1, which is approximately 79 times and 195 times higher than that through pure thermal catalysis and pure photocatalysis, respectively. Moreover, the CO production rate of 7 wt% Ag/AgBr/CsPbBr3 is 7.25 times higher than that of pristine CsPbBr3. The mutual promotion of the thermochemical and photochemical processes significantly improves the energy utilization efficiency and reaction kinetics, leading to high photothermal catalytic efficiency. This work indicates that the inorganic halide perovskite shows excellent photothermal catalytic activity, and provides an alternative way to extend the application range of perovskite materials.


 Please wait while we load your content...
Please wait while we load your content...