Electrochemical ruthenium-catalysed C–H activation in water through heterogenization of a molecular catalyst†
Abstract
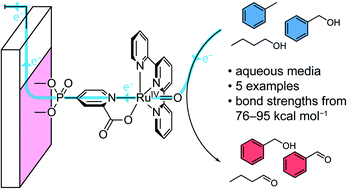
Efficient catalytic oxidative C–H activation of organic substrates remains an important challenge in synthetic chemistry. Here, we show that the combination of a transition metal catalyst, surface immobilisation and an electrochemical potential provide a promising approach to effecting these transformations in aqueous solution. A ruthenium-based molecular catalyst [Ru(tpy)(pic-PO3H2)(Cl)] (where tpy is 2,2′:6′,2′′-terpyridine, pic-PO3H2 is 4-phosphonopyrid-2-ylcarboxylic acid) was synthesised and fully characterised. Oxidation of benzyl alcohol with the catalyst in aqueous media using ceric ammonium nitrate as terminal oxidant resulted in a rapid deactivation of the catalyst. Immobilisation of the catalyst on a mesoporous indium tin oxide electrode surface through the phosphonate anchoring group was shown to circumvent the issues observed in solution. Using the heterogeneous catalyst system, the oxidation of a variety of organic substrates with varying bond dissociation energies was demonstrated with turnover numbers of up to 346. Finally, surface-analysis of the functionalised electrodes after catalysis revealed that fragmentation of the complex during the reaction was the limiting factor for catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...