Mechanism, reactivity, and selectivity in a palladium-catalyzed organosilicon-based cross coupling reaction†
Abstract
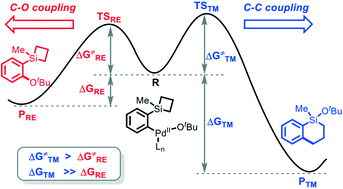
A comprehensive density functional theory (DFT) study has been performed to elucidate the mechanistic details of the Pd-catalyzed strain-release organosilicon based cross coupling reaction of four- and five-membered silacycles. The reaction is proposed to proceed through Ar–Br oxidative addition (OA), Br-to-tBuO ligand exchange (LE), transmetalation, and reductive elimination (RE) steps, and among which the transmetalation is the rate-determining step. Mechanistically, after ligand exchange, the reaction would lead to two distinct pathways: C–O RE to form the C–O coupling product or transmetalation to provide the C–C coupling product, in which the former is often found to be favorable in many other transformations. The experimentally observed uncommon C–C coupling selectivity over the C–O RE in the title reaction is elucidated by an unusual “thermodynamic control scenario”, that is, although the C–O RE has a slightly lower barrier than the transmetalation, however, the product of the transmetalation is much more stable compared to that of the C–O RE. The computational results from different density functional methods and the experimental details verified our findings. The steric effect in the rate-determining step accounts for the contrasting reactivity with different nucleophiles in the reactions with silacyclopentane. The amine base was found to facilitate the transmetalation process by forming a penta-coordinated silacycle. We predict that the −NMe2 containing base would further promote the reaction by decreasing the activation barrier.


 Please wait while we load your content...
Please wait while we load your content...