Mechanistic insight into construction of axially chiral biaryls via palladium/chiral norbornene cooperative catalysis: a DFT-based computational study†
Abstract
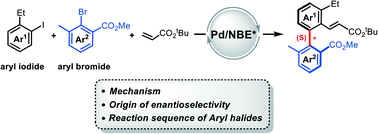
Density functional theory calculations were performed on a prototype of three-component reactions involving aryl iodides, 2,6-substituted aryl bromides, and acrylates to understand the construction of axially chiral biaryls through the palladium/chiral norbornene cooperative catalysis (Nat. Catal.2020, 3, 727–733). The reaction is established to occur in sequence through oxidative addition of aryl iodide to Pd(0), norbornene insertion, C–H activation, oxidative addition of aryl bromide to Pd(II), C–C reductive elimination, norbornene extrusion, alkene insertion, and β-H elimination. The C–H activation is characterized as the rate-determining step, and the oxidative addition of aryl bromide on Pd(II) control the enantioselectivity of the reaction. The construction of more favorable (S)-C–C axial chirality is attributed to the smaller steric repulsion between the ortho-methyl group of aryl bromide and the norbornene moiety. The theoretical results rationalize the reaction sequence of aryl iodide and aryl bromide on both Pd(0) and Pd(II) and clarify the substantial role of the ortho-ester group in the aryl bromide.


 Please wait while we load your content...
Please wait while we load your content...