Effects of potassium on propylene epoxidation by molecular oxygen on Cu2O (111): a DFT study†
Abstract
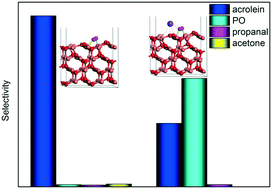
Propylene epoxidation catalyzed by cuprous oxide (Cu2O) with molecular oxygen is significant in the industrial field, and strategies to improve the selectivity of the target product propylene oxide (PO) are highly desired. In the present work, spin-polarized density functional theory (DFT) calculations with Hubbard U correction were employed to investigate the effects of potassium on propylene epoxidation by molecular oxygen on a Cu2O (111) surface. The mechanism for propylene epoxidation can adopt two parallel pathways, an allylic hydrogen stripping (AHS) process and an epoxidation process, and acrolein, PO, propanal, and acetone can be generated. The results calculated here indicated that the valence of the absorbed O2 on the Cu2O (111) surface could be altered by the presence of potassium (K), and the absorbed O2 can be identified as a peroxygen anion (O22−) on a K-modified Cu2O (111) surface. For the primary chemistry of the propylene epoxidation mechanism, it was shown that the AHS pathway was hindered upon the addition of potassium due to the strong K–O bond as well as the steric effects of potassium, thus favoring the epoxidation process. From analysis of the energetic factor for controlling PO and acetone formation (i.e., secondary chemistry in the propylene epoxidation mechanism), it can be found that the binding strength difference between the C2H5OO˙ and 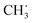 species (EOOC2H5 − ECH3) had an increased effect because of the adjacency of K, which is available to improve the selectivity of PO. The effective free energy barriers for acrolein, PO, propanal, and acetone formation are 1.07, 1.05, 1.32, and 2.08 eV, respectively, on the K-modified Cu2O (111) surface, whereas they are 0.43, 0.66, 0.69, and 0.62 eV, respectively, on a pure Cu2O (111) surface, indicating that the PO formation selectivity increased in the presence of K, although its activity is decreased to some extent. Moreover, microkinetic analysis was carried out to simulate propylene epoxidation on a Cu2O (111) surface with and without K modification, and the results demonstrated that the crucial, competitive step is the first H-stripping step in the AHS process on the Cu2O (111) surface, and the rate-determining step is the generation of OOMMP2 on the K-modified Cu2O (111) surface for the formation of PO. From this study, it was found that the selectivity of PO can be improved upon the use of an alkali metal promoter, either via decreasing the catalytic activity of the first H-stripping step (reducing the oxygen basic strength) or via increasing the oxametallacycle formation activity.
species (EOOC2H5 − ECH3) had an increased effect because of the adjacency of K, which is available to improve the selectivity of PO. The effective free energy barriers for acrolein, PO, propanal, and acetone formation are 1.07, 1.05, 1.32, and 2.08 eV, respectively, on the K-modified Cu2O (111) surface, whereas they are 0.43, 0.66, 0.69, and 0.62 eV, respectively, on a pure Cu2O (111) surface, indicating that the PO formation selectivity increased in the presence of K, although its activity is decreased to some extent. Moreover, microkinetic analysis was carried out to simulate propylene epoxidation on a Cu2O (111) surface with and without K modification, and the results demonstrated that the crucial, competitive step is the first H-stripping step in the AHS process on the Cu2O (111) surface, and the rate-determining step is the generation of OOMMP2 on the K-modified Cu2O (111) surface for the formation of PO. From this study, it was found that the selectivity of PO can be improved upon the use of an alkali metal promoter, either via decreasing the catalytic activity of the first H-stripping step (reducing the oxygen basic strength) or via increasing the oxametallacycle formation activity.


 Please wait while we load your content...
Please wait while we load your content...