Hydrodeoxygenation of guaiacol over orthorhombic molybdenum carbide: a DFT and microkinetic study†
Abstract
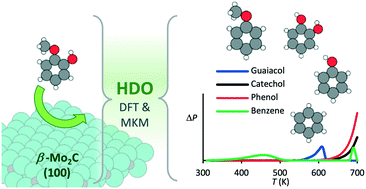
The hydrodeoxygenation of guaiacol is modelled over a (100) β-Mo2C surface using density functional theory and microkinetic simulations. The thermochemistry of the process shows that the demethoxylation of the guaiacol, to form phenol, will be the initial steps, with a reaction energy of 29 kJ mol−1 (i.e. endothermic) and a highest activation barrier of 112 kJ mol−1. Subsequently, the dehydroxylation of the phenol, which has a rate-determining activation barrier of 145 kJ mol−1, will lead to the formation of benzene, with an overall reaction energy for conversion from guaiacol of −91 kJ mol−1 (i.e. exothermic). The sp2 and sp hybridized carbon atoms of the molecular functional groups are found to dissociate on the surface with minimum energy barriers, while the hydrogenation of the adsorbed molecules requires higher energy. The microkinetic modelling, which is performed considering typical reaction conditions of 500 to 700 K, and a partial pressure ratio for H2 : guaiacol of 1, shows quick formation and accumulation of phenol on the surface with increasing temperature, although high temperatures mitigate the guaiacol adsorption step. Based on simulated temperature programmed desorption (TPD), maximum conversion of guaiacol can be expected at 70% surface coverage of this species.


 Please wait while we load your content...
Please wait while we load your content...