Nonplanar porphyrins: synthesis, properties, and unique functionalities
Abstract
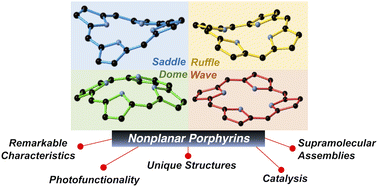
Porphyrins are variously substituted tetrapyrrolic macrocycles, with wide-ranging biological and chemical applications derived from metal chelation in the core and the 18π aromatic surface. Under suitable conditions, the porphyrin framework can deform significantly from regular planar shape, owing to steric overload on the porphyrin periphery or steric repulsion in the core, among other structure modulation strategies. Adopting this nonplanar porphyrin architecture allows guest molecules to interact directly with an exposed core, with guest-responsive and photoactive electronic states of the porphyrin allowing energy, information, atom and electron transfer within and between these species. This functionality can be incorporated and tuned by decoration of functional groups and electronic modifications, with individual deformation profiles adapted to specific key sensing and catalysis applications. Nonplanar porphyrins are assisting breakthroughs in molecular recognition, organo- and photoredox catalysis; simultaneously bio-inspired and distinctly synthetic, these molecules offer a new dimension in shape-responsive host–guest chemistry. In this review, we have summarized the synthetic methods and design aspects of nonplanar porphyrin formation, key properties, structure and functionality of the nonplanar aromatic framework, and the scope and utility of this emerging class towards outstanding scientific, industrial and environmental issues.
- This article is part of the themed collection: Trends and Challenges in Porphyrinoid Chemistry


 Please wait while we load your content...
Please wait while we load your content...