The impact of the cation alkyl chain length on the wettability of alkylimidazolium-based ionic liquids at the nanoscale†
Abstract
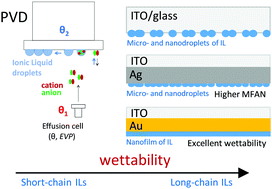
Ionic liquids (ILs) have been widely used for energy storage and conversion devices due to their negligible vapor pressure, high thermal stability, and outstanding interfacial properties. Notably, the interfacial nanostructure and the wettability of thin ionic liquid films on solid surfaces are of utmost relevance in nanosurface science and technology. Herein, a reproducible physical vapor deposition methodology was used to fabricate thin films of four alkylimidazolium bis(trifluoromethylsulfonyl)imide ILs. The effect of the cation alkyl chain length on the wettability of ILs was explored on different surfaces: gold (Au); silver (Ag); indium-tin oxide (ITO). High-resolution scanning electron microscopy (SEM) and atomic force microscopy (AFM) were used to evaluate the morphology of the produced micro- and nanodroplets and films. SEM and AFM results revealed an island growth for all the ILs deposited on ITO and Ag surfaces, with a lower minimum free area to promote nucleation (MFAN) in Ag and higher wettability for ILs having larger non-polar domains. The low wettability of ITO by the studied ILs was highlighted. For long-chain ILs, nucleation and growth mechanisms were strongly conditioned by coalescence processes. The results also supported the higher affinity of the ILs to the Au surface. The increase in the length of the cation alkyl chain was found to promote a better film adhesion inducing a 2D growth and higher wetting ability.


 Please wait while we load your content...
Please wait while we load your content...