Plasma-promoted reactions of the heterobimetallic anions CuNb− with dinitrogen and subsequent reactions with carbon dioxide: formation of C–N bonds†
Abstract
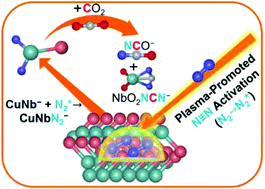
The activation and functionalization of dinitrogen with carbon dioxide into useful chemicals containing C–N bonds are significant research projects but highly challenging. Herein, we report that N2 molecules are dissociated by heterobimetallic CuNb− anions assisted by surface plasma radiation, leading to the formation of CuNbN2− anions; the CuNbN2− anions can further react with CO2 to generate products NCO− with one C–N bond and NbO2NCN− with two C–N bonds under thermal collision conditions. For the activation of dinitrogen, the plasma atmosphere is conducive to the dissociation of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, which renders the coupling reactions of N2 and CO2 molecules easier to proceed. This is the first report of coupling of N2 and CO2 to generate C–N bonds by making good use of the plasma effect to assist in the activation of N2 molecules. This new strategy with the assistance of plasma provides a practicable route to construct C–N bonds by directly using N2 and CO2 at room temperature.
N bond, which renders the coupling reactions of N2 and CO2 molecules easier to proceed. This is the first report of coupling of N2 and CO2 to generate C–N bonds by making good use of the plasma effect to assist in the activation of N2 molecules. This new strategy with the assistance of plasma provides a practicable route to construct C–N bonds by directly using N2 and CO2 at room temperature.


 Please wait while we load your content...
Please wait while we load your content...