Chemical potential, derivative discontinuity, fractional electrons, jump of the Kohn–Sham potential, atoms as thermodynamic open systems, and other (mis)conceptions of the density functional theory of electrons in molecules
Abstract
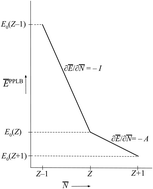
Many references exist in the density functional theory (DFT) literature to the chemical potential of the electrons in an atom or a molecule. The origin of this notion has been the identification of the Lagrange multiplier μ = ∂E/∂N in the Euler–Lagrange variational equation for the ground state density as the chemical potential of the electrons. We first discuss why the Lagrange multiplier in this case is an arbitrary constant and therefore cannot be a physical characteristic of an atom or molecule. The switching of the energy derivative (“chemical potential”) from −I to −A when the electron number crosses the integer, called integer discontinuity or derivative discontinuity, is not physical but only occurs when the nonphysical noninteger electron systems and the corresponding energy and derivative ∂E/∂N are chosen in a specific discontinuous way. The question is discussed whether in fact the thermodynamical concept of a chemical potential can be defined for the electrons in such few-electron systems as atoms and molecules. The conclusion is that such systems lack important characteristics of thermodynamic systems and do not afford the definition of a chemical potential. They also cannot be considered as analogues of the open systems of thermodynamics that can exchange particles with an environment (a particle bath or other members of a Gibbsian ensemble). Thermodynamical (statistical mechanical) concepts like chemical potential, open systems, grand canonical ensemble etc. are not applicable to a few electron system like an atom or molecule. A number of topics in DFT are critically reviewed in light of these findings: jumps in the Kohn–Sham potential when crossing an integer number of electrons, the band gap problem, the deviation-from-straight-lines error, and the role of ensembles in DFT.
- This article is part of the themed collections: 2022 PCCP HOT Articles and PCCP Reviews


 Please wait while we load your content...
Please wait while we load your content...