Efficient synthesis of cyclic carbonates from CO2 under ambient conditions over Zn(betaine)2Br2: experimental and theoretical studies†
Abstract
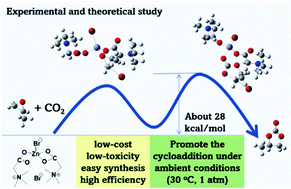
It is very interesting to synthesize high value-added chemicals from CO2 under mild conditions with low energy consumption. Here, we report that a novel catalyst, Zn(betaine)2Br2, can efficiently promote the cycloaddition of CO2 with epoxides to synthesize cyclic carbonates under ambient conditions (30 °C, 1 atm). DFT calculations provide important insights into the mechanism, particularly the unusual synergistic catalytic action of Zn2+, Br− and NR4+, which is the critical factor for the outstanding performance of Zn(betaine)2Br2. The unique features of the catalyst are that it is cheap, green and very easy to prepare.


 Please wait while we load your content...
Please wait while we load your content...