Role of post-CCSD(T) corrections in predicting the energetics and kinetics of the OH˙ + O3 reaction†
Abstract
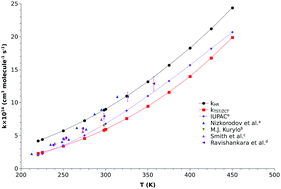
The present work investigates the OH˙ +O3 reaction by means of chemical kinetics and quantum chemical calculations. To estimate the reaction barrier height and reaction energy, we have included various corrections, like zero point energy corrections, contribution from full triple excitations and partial quadratic excitations at the coupled-cluster level, core corrections, diagonal Born–Oppenheimer corrections and spin–orbit coupling corrections. The reaction barrier and reaction energy were estimated to be 1.64 kcal mol−1 and −39.97 kcal mol−1, respectively, which are in good agreement with the experimental results. Using this barrier height, we have estimated the rate constants using transition state theory augmented with zero curvature tunneling (TST/ZCT) in the temperature range of 220–450 K and the thus obtained rate constants were compared with available experimental results.


 Please wait while we load your content...
Please wait while we load your content...