Investigating the competing E2 and SN2 mechanisms for the microsolvated HO−(H2O)n=0–4 + CH3CH2X (X = Cl, Br, I) reactions†
Abstract
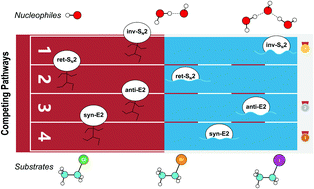
We characterized the anti-E2, syn-E2, inv-SN2, and ret-SN2 reaction channels for the reaction of microsolvated HO−(H2O)n anions with CH3CH2X (X = Cl, Br, I), using the CCSD(T)/PP/t//MP2/ECP/d level method, to understand how a solvent influences the competing E2 and SN2 reactions. The calculated sequence of barrier for the four channels is ret-SN2 > syn-E2 > anti-E2 > inv-SN2. The barrier heights increase with incremental hydration as the system transfers from the gas phase to microsolvation, and to bulk solvation (using the PCM implicit solvent model). As the degree of hydration n increases, good correlations have been found between barrier heights and several thermodynamic, geometric and charge parameters, including the reaction enthalpy, proton/ethyl-cation affinity of the hydrated nucleophile, geometric looseness (%L‡) and asymmetry (%AS‡) and charge asymmetry (Δq(X–O)) of the transition structures. Under a molecular orbital scheme, the HOMOs of nucleophiles are stabilized by stepwise hydration, explaining the rise in the barriers. Considering the effect of the leaving group, the barrier heights exhibit linear correlation with the halogen electronegativity and H-acidity of substrate CH3CH2X. In terms of E2/SN2 competition, the barrier difference, 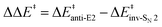 , first increases then decreases as the number of explicit water molecules increases, under both microsolvation and bulk solvation conditions, but the inv-SN2 pathway is always favored over the anti-E2 pathway. Energy decomposition analysis attributes the increase of barrier difference to the greater geometric distortion in the anti-E2 transition structure.
, first increases then decreases as the number of explicit water molecules increases, under both microsolvation and bulk solvation conditions, but the inv-SN2 pathway is always favored over the anti-E2 pathway. Energy decomposition analysis attributes the increase of barrier difference to the greater geometric distortion in the anti-E2 transition structure.


 Please wait while we load your content...
Please wait while we load your content...