Hydrogen and halogen bond synergy in the self-assembly of 3,5-dihalo-tyrosines: structural and theoretical insights†
Abstract
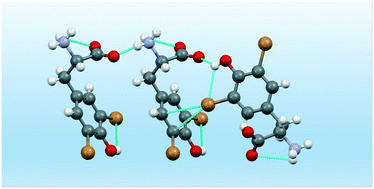
Halogenation, generally introduced on aromatic amino acids, is becoming a key supramolecular tool in peptides. Herein, we report the crystal structures and DFT study of two bis-halogenated tyrosines showing the subtle relationship between hydrogen and halogen bonds in promoting their supramolecular self-assembly.
- This article is part of the themed collection: New Talent 2022


 Please wait while we load your content...
Please wait while we load your content...