Multiple crystallization pathways of amorphous calcium carbonate in the presence of poly(aspartic acid) with a chain length of 30†
Abstract
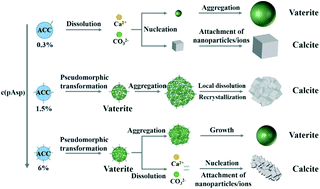
The crystallization pathways of amorphous calcium carbonate (ACC) have attracted tremendous interest because of the importance of ACC in biomineralization. Here, by using poly(aspartic acid) with a chain length of 30 (pAsp-30) as an additive, we show multiple crystallization pathways of ACC depending on the concentration of pAsp-30. Although ACC transforms into a mixture of calcite and vaterite via a typical dissolution recrystallization mechanism at low concentration of pAsp-30, a pseudomorphic transformation from ACC into pure vaterite was observed at intermediate concentration of pAsp-30. These vaterite nanoparticles then aggregated and transformed into pure calcite by a local dissolution recrystallization mechanism. Further increasing the concentration of pAsp-30 inhibited the aggregation of vaterite nanoparticles and led to the formation of a mixture of calcite and vaterite again. Additionally, the formation of both vaterite and calcite by a particle attachment crystallization mechanism was also observed. By simply changing the concentration of pAsp-30, delicate control of the polymorph selection between pure calcite and pure vaterite can be easily achieved. These results significantly improve our understanding of the crystallization mechanism of ACC and the role of additives in controlling the crystallization pathways.


 Please wait while we load your content...
Please wait while we load your content...