Versatility in self-assembly and morphology of non-coded anthranilic acid and phenylglycine based dipeptide stereoisomers†
Abstract
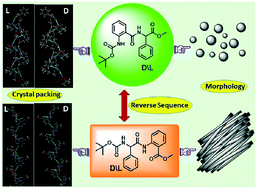
Beauty in the self-assembly patterns of isomeric dipeptides of Boc-Ant-L-Phg-OMe (1) bearing two rigid, unnatural amino acids (Ant: anthranilic acid, Phg: phenylglycine) is demonstrated. Additionally, self-assembly and morphological variation by the incorporation of a D-amino acid, Boc-Ant-D-Phg-OMe (2), and corresponding reversed sequences of both peptides, Boc-L-Phg-Ant-OMe (3) and Boc-D-Phg-Ant-OMe (4), respectively, are explored. FT-IR study indicated the presence of conformational heterogeneity in the reverse peptides. SC-XRD suggested that 1 and 2 contain helical and β sheet-like layer architectures, respectively, whereas 3 and 4 exhibited different kinds of helical structures, sheet-like layer architectures, and molecular channels. Optical microscopy, FESEM, FETEM, and AFM images suggested that both C-terminal L- and D-Phg containing peptides (1 and 2) self-assembled to form a vesicular morphology and their reversed sequences, i.e., N-terminal L- and D-Phg containing peptides (3 and 4), displayed a well-organized rod-like fiber structure. TGA analysis revealed that the obtained supramolecular structures have significant thermal stability.


 Please wait while we load your content...
Please wait while we load your content...