Asymmetric addition of an N-methyl C(sp3)–H bond to cyclic alkenes enabled by an iridium/phosphine–olefin catalyst†
Abstract
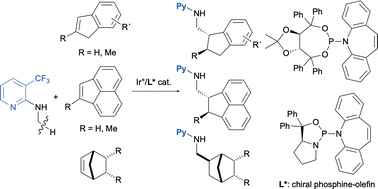
Iridium-catalyzed asymmetric C–H addition of an N-methyl group on 3-trifluoromethyl-2-(N-methylamino)pyridine to cyclic alkenes was realized by using a phosphine–olefin ligand. Indene and its 7-substituted derivatives, acenaphthylene, and some bicyclic alkenes underwent the addition to give the corresponding products in good yields with high enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...