Catalytic (3 + 2) umpolung annulations of α-thioacyl carbenes with aryl isothiocyanates†
Abstract
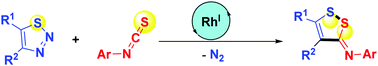
1,2,3-Thiadiazoles serve as masked S-electrophilic thia-1,3-dipoles. Under rhodium/racemic BINAP catalysis, they undergo denitrogenative (3 + 2) umpolung transannulations with aryl isothiocyanates with inverse regioselectivity and excellent stereoselectivity, yielding N-aryl 3H-1,2-dithiol-(Z)-3-imines in a redox-neutral, step-efficient, and functionality-tolerant manner. An intramolecular S–S bond is impressively forged.


 Please wait while we load your content...
Please wait while we load your content...