Gold-catalyzed redox cycloisomerization/nucleophilic addition/reduction: direct access to 2-phosphoryl indolin-3-ones†
Abstract
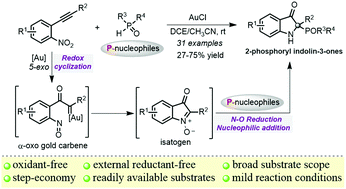
An efficient gold(I)-catalyzed redox cycloisomerization/nucleophilic addition/reduction reaction of o-nitroalkynes with various H-phosphorus oxides is established. Through the intramolecular redox cyclization of o-nitroalkynes and subsequent intermolecular nucleophilic addition/reduction with no external reactant, a variety of arylphosphoryl and alkylphosphoryl indolin-3-ones with high functional-group compatibility are obtained in moderate to good yields. Mechanistic studies suggest that phosphorus nucleophiles mediate the cleavage of the N–O bond as a reductant.


 Please wait while we load your content...
Please wait while we load your content...