KOtBu-promoted C3-homocoupling of quinoxalinones through single electron transfer from an sp2 carbanion intermediate†
Abstract
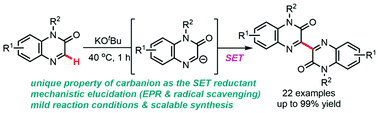
The C3-selective homodimerization of quinoxalinones is described. A C3-sp2 carbanion species generated through deprotonation of quinoxalinone using potassium tert-butoxide (KOtBu) transfers an electron (single electron transfer mechanism) to a second quinoxalinone, affording a radical–anion intermediate. The radical scavenging and electron paramagnetic resonance (EPR) experiments support the plausible radical reaction pathway. A mild reaction temperature and a short reaction time were attained under cost-effective conditions, which reveal the amenability of this protocol to pharmaceutical and chemical industries.


 Please wait while we load your content...
Please wait while we load your content...