Novel cinnamic acid analogues: synthesis of aminotroponyl acrylates by Pd(ii)-catalysed C(sp2)–H olefination†
Abstract
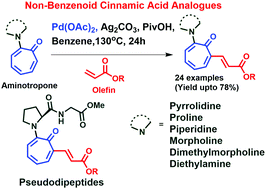
Cinnamic acid, a benzenoid scaffold, is a building block of various natural products. This report describes the synthesis of new non-benzenoid cinnamate analogs, 3-(6-amino-7-oxocyclohepta-1,3,5-trien-1-yl)acrylates, obtained through Pd(II)-catalyzed C7-H olefination of 2-aminotropones in the presence of acrylates. In these site-selective couplings, the troponyl-carbonyl function acts as a directing group. This strategy has been employed for the synthesis of new pseudopeptides from prolyl/prolamide containing aminotropone derivatives. These novel troponyl cinnamate analogs are potential precursors of hairpin forming peptides.


 Please wait while we load your content...
Please wait while we load your content...