Aromaticity-enhanced reactivity of geminal frustrated Lewis pairs†
Abstract
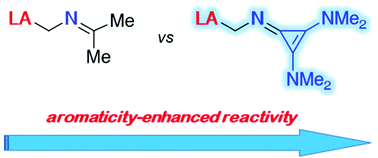
The presence of a cyclopropenimine moiety as the Lewis base partner in geminal frustrated Lewis pairs greatly enhances the reactivity of the system towards the activation of small molecules. This is mainly due to an increase of the aromaticity strength of this fragment during the activation reaction which results in a significant gain of stability ultimately leading to low barrier and high exergonic transformations.


 Please wait while we load your content...
Please wait while we load your content...