Selective benzylic Csp3–H bond activations mediated by a phosphorus–nitrogen PN3P-nickel complex†
Abstract
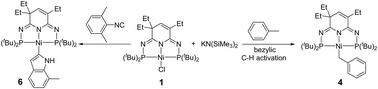
In contrast to the typical Csp2–H activation, a PN3P-Nickel complex chemoselectively cleaved the benzylic Csp3–H bond of toluene in the presence of KHMDS, presumably via an in situ generated potassium benzyl intermediate. Under similar conditions, CO underwent deoxygenation to afford the corresponding nickel cyano complex, and ethylbenzene was dehydrogenated to give styrene and a nickel hydride compound. 2,6-Xylyl isocyanide was transformed into an unprecedented indolyl complex, likely by trapping the activated benzyl species with an isocyanide moiety.


 Please wait while we load your content...
Please wait while we load your content...