A new magnetic ionic liquid based salting-out assisted dispersive liquid–liquid microextraction for the determination of parabens in environmental water samples
Abstract
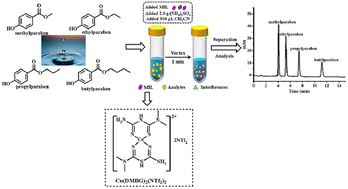
In this study, a new magnetic ionic liquid (MIL) was designed and prepared, containing a magnetic cation from the ligand N,N-dimethyl biguanide (DMBG) complexing with magnetic center Co2+ and a bis-trifluoromethanesulfonimide (NTf2−) anion. Using the MIL as the extraction solvent, a salting-out assisted dispersive liquid–liquid microextraction (SA-DLLME) combined with high performance liquid chromatography-ultraviolet detection (HPLC-UV) was established for the enrichment and detection of four parabens in environmental water samples. The one-factor-at-a-time experiment was employed to optimize the conditions affecting the extraction efficiency. Under the optimized extraction conditions, the limits of quantification (LOQs) of the four target analytes ranged from 2.0 ng mL−1 to 2.8 ng mL−1, and the coefficients of determination (R2) were above 0.9996 in the linear range of 2.8–400 ng mL−1. On the other hand, the method displayed good repeatability and accuracy with intra-day and inter-day relative standard deviations (RSDs) of 2.1–13.0% and recoveries of 82.0–114.6%. The established method was applied to real samples with recoveries within 81.6–125.4%, and the results demonstrated that the method was practical.


 Please wait while we load your content...
Please wait while we load your content...