A novel ratiometric fluorescent and colorimetric sensor based on a 1,8-naphthalimide derivative for nanomolar Cu2+ sensing: smartphone and food applications†
Abstract
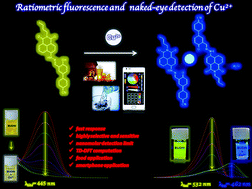
A novel 1,8-naphthalimide-based chemical sensor 3-((2-(2-butyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinoline-6-yl)hydrazone-o)methyl)-4-hydroxy-[1,1-biphenyl]-4-carbonitrile (BUDIN) with ratiometric fluorescence behavior, as well as “naked-eye” response was developed for the sensitive and specific determination of Cu2+ at nanomolar levels. With the addition of different amounts of Cu2+, the emission of BUDIN varied continually, leading to colour changes from yellow to blue (λem = 532 nm to 462 nm) due to intra-ligand charge transfer (ILCT) and metal–ligand charge transfer (MCLT). BUDIN can detect Cu2+ quantitatively with a detection limit of 17 nM. Job's plot, MALDI TOF MS and TOF MS findings revealed that the binding stoichiometry of BUDIN and Cu2+ was 2 : 1. Furthermore, the theoretical computation data strongly supported the optical response of BUDIN toward Cu2+. Smartphone digital imaging studies proved that BUDIN can be utilized as an outstanding chemical sensor for the on-site detection of Cu2+ without the need for sophisticated equipment, with a detection limit of 17.8 μM. The findings also presented that BUDIN is a very effective chemosensor for Cu2+ in real food samples with quite a simple operation.
- This article is part of the themed collection: Analyst HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...