Development of a novel chromophore reaction-based fluorescent probe for biogenic amines detection†
Abstract
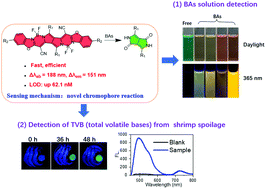
Biogenic amines (BAs) are important biomarkers to monitor meat spoilage. However, the design of efficient BA fluorescent probes with distinct colorimetric and ratiometric fluorescent dual-channels is still a critical challenge because of similar chemical properties and basicity between BAs and other amines. Herein, pyrrolopyrrole cyanine (PPCy-1) is reported to display distinctly high reactivity toward BAs through an ultrasensitive irreversible chromophore reaction for the first time. The reaction mechanism is ascribed to synergistic aza-Michael addition and B–N detachment, followed by hydrolysis to produce low-conjugated diketopyrrolopyrrole and heteroaromatic acetonitrile compounds. As a result, colorimetric and ratiometric fluorescent dual-channel (Δλab = 188 nm and Δλem = 151 nm) signals and a limit of detection up to 62.1 nM level for BA solution are acquired. In addition, the colorimetric detection of volatile amine vapor using the PPCy-1-loaded filter paper, showing a color change from green to yellow, is feasible. A simple and cost-effective fluorescence “turn on” method using the filter paper or the CAD-40 resin loaded with PPCy-1 to detect TVB (total volatile bases) originating from shrimp spoilage is further demonstrated.
- This article is part of the themed collections: Celebrating International Women’s Day: Women in Materials Science, 2021 Journal of Materials Chemistry B most popular articles and Journal of Materials Chemistry B Lunar New Year collection 2022


 Please wait while we load your content...
Please wait while we load your content...