Cationic helicenes as selective G4 DNA binders and optical probes for cellular imaging†
Abstract
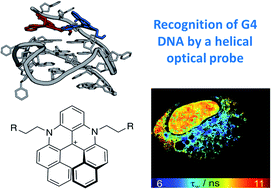
The important role that G-quadruplex DNA (G4 DNA) structures play in regulating biological processes is becoming widely recognised. These structures have also been proposed to be attractive drug targets. Therefore, there has been significant interest in developing small molecules that can selectively bind to G4 DNA over other topologies. In this paper we investigate the interaction between DNA and helical compounds (helicenes) based on a central carbocation trisubstituted with aromatic rings. We show that the non-planar structure of these helicenes results in a significantly reduced affinity for dsDNA when compared to their planar analogues, whilst maintaining a high affinity for G4 DNA. Additionally, the right- and left-handed enantiomers of one of these helicenes recognise the chiral DNA environments of G4 and dsDNA differently. We show that upon DNA binding the helicenes display a fluorescence switch-on effect, which we have successfully used for cellular imaging in live and fixed U2OS cells, staining mitochondria and the nucleus, respectively.


 Please wait while we load your content...
Please wait while we load your content...