Kinetic resolution of sulfur-stereogenic sulfoximines by Pd(ii)–MPAA catalyzed C–H arylation and olefination†
Abstract
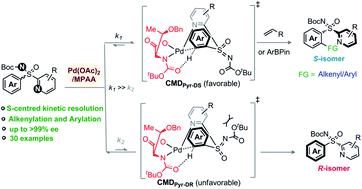
A direct Pd(II)-catalyzed kinetic resolution of heteroaryl-enabled sulfoximines through an ortho-C–H alkenylation/arylation of arenes has been developed. The coordination of the sulfoximine pyridyl-motif and the chiral amino acid MPAA ligand to the Pd(II)-catalyst controls the enantio-discriminating C(aryl)–H activation. This method provides access to a wide range of enantiomerically enriched unreacted aryl-pyridyl-sulfoximine precursors and C(aryl)–H alkenylation/arylation products in good yields with high enantioselectivity (up to >99% ee), and selectivity factor up to >200. The coordination preference of the directing group, ligand effect, geometry constraints, and the transient six-membered concerted-metalation–deprotonation species dictate the stereoselectivity; DFT studies validate this hypothesis.
- This article is part of the themed collection: Celebrating five years of ChemRxiv


 Please wait while we load your content...
Please wait while we load your content...