A short, versatile route towards benzothiadiazinyl radicals†
Abstract
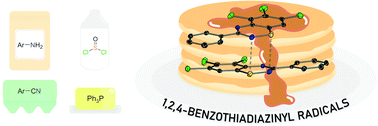
A family of substituted 1,2,4-benzothiadiazine 1-chlorides have been prepared by treatment of N-arylamidines in neat thionyl chloride at reflux. The S(IV) 1-chlorides are readily reduced under mild conditions to persistent 1,2,4-benzothiadiazinyl radicals which have been characterised by EPR spectroscopy and cyclic voltammetry. Crystallographic studies on isolated radicals indicate that the radicals dimerise via pancake bonding in the solid-state, resulting in spin-pairing and net diamagnetism.


 Please wait while we load your content...
Please wait while we load your content...