Me3SiSiMe2(OnBu): a disilane reagent for the synthesis of diverse silacycles via Brook- and retro-Brook-type rearrangement†
Abstract
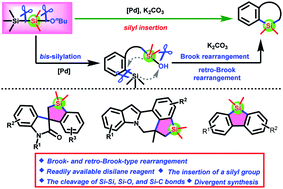
Herein, a readily available disilane Me3SiSiMe2(OnBu) has been developed for the synthesis of diverse silacycles via Brook- and retro-Brook-type rearrangement. This protocol enables the incorporation of a silylene into different starting materials, including acrylamides, alkene-tethered 2-(2-iodophenyl)-1H-indoles, and 2-iodobiaryls, via the cleavage of Si–Si, Si–C, and Si–O bonds, leading to the formation of spirobenzosiloles, fused benzosiloles, and π-conjugated dibenzosiloles in moderate to good yields. Preliminary mechanistic studies indicate that this transformation is realized by successive palladium-catalyzed bis-silylation and Brook- and retro-Brook-type rearrangement of silane-tethered silanols.


 Please wait while we load your content...
Please wait while we load your content...