Accelerating the insertion reactions of (NHC)Cu–H via remote ligand functionalization†
Abstract
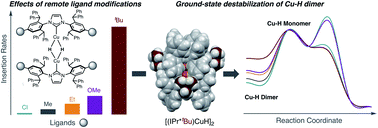
Most ligand designs for reactions catalyzed by (NHC)Cu–H (NHC = N-heterocyclic carbene ligand) have focused on introducing steric bulk near the Cu center. Here, we evaluate the effect of remote ligand modification in a series of [(NHC)CuH]2 in which the para substituent (R) on the N-aryl groups of the NHC is Me, Et, tBu, OMe or Cl. Although the R group is distant (6 bonds away) from the reactive Cu center, the complexes have different spectroscopic signatures. Kinetics studies of the insertion of ketone, aldimine, alkyne, and unactivated α-olefin substrates reveal that Cu–H complexes with bulky or electron-rich R groups undergo faster substrate insertion. The predominant cause of this phenomenon is destabilization of the [(NHC)CuH]2 dimer relative to the (NHC)Cu–H monomer, resulting in faster formation of Cu–H monomer. These findings indicate that remote functionalization of NHCs is a compelling strategy for accelerating the rate of substrate insertion with Cu–H species.


 Please wait while we load your content...
Please wait while we load your content...