Library construction of stereochemically diverse isomers of spirooliganin: their total synthesis and antiviral activity†
Abstract
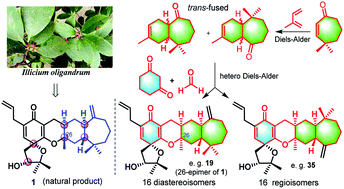
The construction of libraries of stereoisomers of natural products serves as an important approach to investigating the correlation between the stereostructure and biological activity. However, the total synthesis and isomerzation of polycyclic scaffolds with multiple chrial centers are rare. Spirooliganin (1), a new skeleton natural product isolated from the plant Illicium oligandrum, was structurally characterized by comprehensive analysis of NMR spectroscopic data and ECD which revealed an unprecedented 5–6–6–6–7 polycyclic framework with six chiral centers. Here we report a 17-step total synthesis to prepare a library of stereochemically diverse isomers of spirooliganin, including 16 diastereoisomers and 16 regioisomers. In addition to a regioselective hetero-Diels–Alder cycloaddition, the synthetic strategy involves a photo-induced stereoselective Diels–Alder reaction, which gives only the abnormal trans-fused product as rationalized by density functional theory calculations. Preliminary biological evaluation showed that spirooliganin and regioisomers 39 exhibited potent inhibition of Coxsackievirus B3. It also revealed the pharmacophore effect of the D-ring (16R,18R,24R, and 26R) for their antiviral activities.


 Please wait while we load your content...
Please wait while we load your content...