Harnessing sulfur and nitrogen in the cobalt(iii)-catalyzed unsymmetrical double annulation of thioamides: probing the origin of chemo- and regio-selectivity†
Abstract
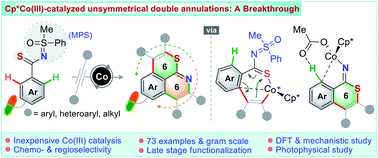
An unconventional cobalt(III)-catalyzed one-pot domino double annulation of aryl thioamides with unactivated alkynes is presented. Sulfur (S), nitrogen (N), and o,o′-C–H bonds of aryl thioamides are involved in this reaction, enabling access to rare 6,6-fused thiopyrano-isoquinoline derivatives. A reverse ‘S’ coordination over a more conventional ‘N’ coordination of thioamides to the Co-catalyst specifically regulates the formation of four [C–C and C–S at first and then C–N and C–C] bonds in a single operation, a concept which is uncovered for the first time. The power of the N-masked methyl phenyl sulfoximine (MPS) directing group in this annulation sequence is established. The transformation is successfully developed, building a novel chemical space of structural diversity (56 examples). In addition, the late-stage annulation of biologically relevant motifs and drug candidates is disclosed (17 examples). The preliminary photophysical properties of thiopyrano-isoquinoline derivatives are discussed. Density functional theory (DFT) studies authenticate the participation of a unique 6π-electrocyclization of a 7-membered S-chelated cobaltacycle in the annulation process.


 Please wait while we load your content...
Please wait while we load your content...