Asymmetric hydroalkylation of alkynes and allenes with imidazolidinone derivatives: α-alkenylation of α-amino acids†
Abstract
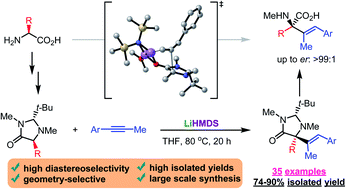
This work reports a new method for the synthesis of quaternary α-alkenyl substituted amino acids by the enantio- and diastereoselective addition of imidazolidinone derivatives to alkynes and allenes. Further hydrolysis of the imidazolidinone products under acidic conditions afforded biologically relevant amino acid derivatives. This method is geometry-selective (E-isomer), enantio- and diastereoselective, and products were obtained in good to excellent yields. The utility of this new methodology is proved by its operational simplicity and the successful accomplishment of gram-scale reactions. Experimental and computational studies suggest the key role of Li in terms of selectivity and support the proposed reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...