Recovery of Zn and Ge from zinc oxide dust by ultrasonic-H2O2 enhanced oxidation leaching
Abstract
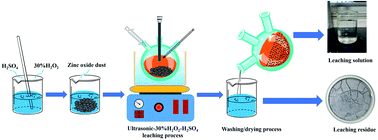
Zn and Ge were selectively extracted from zinc oxide dust (ZOD) by the ultrasonic-H2O2 (UH) combined oxidation-leaching process. In the leaching process, the effects of the dosage of H2O2 (6–29.5 mL), ultrasonic power, initial acidity (100–200 g L−1), liquid/solid mass ratio (4–8 : 1), leaching temperature (50–90 °C), and leaching time (30–240 min) on the leaching rates of Zn and Ge were studied. The experimental results showed that the ultrasonic power and the dosage of H2O2 have the greatest influence on the leaching rates of Zn and Ge. The results showed the optimum conditions as: ultrasonic power 200 W, the dosage of H2O2 14.8 mL, initial acidity 160 g L−1, liquid/solid mass ratio 7 : 1, leaching time 60 min, stirring speed 400 rpm, leaching temperature 60 °C, and the leaching rate of Zn and Ge reaches 99.61% and 88.29%, respectively. The leaching rates of Zn and Ge by UH were 7.86% and 5.65% higher than that by conventional leaching (CL), respectively. The experimental results showed that UH leaching technology can improve the rates of Zn and Ge from ZOD, reduce the leaching temperature, save the production cost, solve the problem of low leaching rates of Zn and Ge in ZOD treatment technology, and realize the resource, reduction and harmless treatment of ZOD.


 Please wait while we load your content...
Please wait while we load your content...