An efficient and practical aerobic oxidation of benzylic methylenes by recyclable N-hydroxyimide†
Abstract
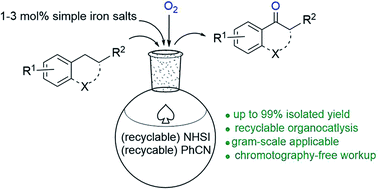
An efficient and practical benzylic aerobic oxidation catalyzed by cheap and simple N-hydroxyimide organocatalyst has been achieved with high yields and broad substrate scope. The organocatalyst used can be recycled and reused by simple workup and only minute amount (1 mol% in most cases) of simple iron salt is used as promoter. Phenyl substrates with mild and strong electron-withdrawing group could also be oxygenated in high yields as well as other benzylic methylenes. Influence of substituents, gram-scale application, catalysts decay and general mechanism of this methodology has also been discussed.


 Please wait while we load your content...
Please wait while we load your content...