Exploration of hydrated lithium manganese oxide with a nanoribbon structure as cathodes in aqueous lithium ion and magnesium ion batteries†
Abstract
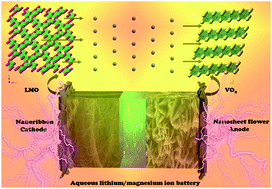
Aqueous batteries with the characteristics of low cost, high safety and environmental friendliness are secondary battery energy storage systems that use inorganic salt solutions as electrolytes. Researchers first explored the aqueous lithium ion battery because of its excellent energy density. Nevertheless, the high cost and limited resources of lithium cannot meet the sustainable development of the goal. Aqueous magnesium ion batteries have received widespread attention due to the similar chemical properties and ionic radius of lithium and magnesium ions. Here, we report a hydrated lithium manganese oxide, Li0.21MnO2·H2O (LMO), with a nanoribbon morphology as a cathode, and compared the electrochemical performance in lithium salt and magnesium salt electrolytes. Moreover, we focused on exploring the changing laws that affect the performance of this electrode in magnesium salt electrolyte. We also prepared VO2 nanosheet flower as the anode, showing excellent electrochemical properties in the same electrolytes. Finally, we assembled an aqueous magnesium ion full battery by using LMO as the cathode and VO2 as the anode, which showed outstanding rate ability. The discharge capacities of this device at the current densities of 20, 50, 100, 200, and 300 mA g−1 are 74.8, 54.7, 46.4, 34.0, and 32.1 mA h g−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...