An inorganic Co-containing heteropolyoxoniobate: reversible chemochromism and H2O-dependent proton conductivity properties†
Abstract
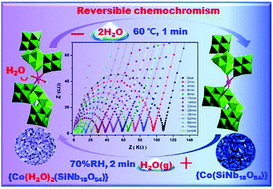
A novel pure inorganic cobalt-containing hetero-polyoxoniobate H9KNa2(H2O)10[Co(H2O)2(SiNb18O54)]·15H2O (1-purple) has been constructed from crescent-shaped [SiNb18O54]14− units. In compound 1-purple, the [SiNb18O54]14− units are connected by [Co(H2O)2]2+ ions to form a chiral helical chain. The adjacent helical chains with left/right-handedness are bridged by K+ ions to generate a 2D inorganic layered structure, where the water molecules were trapped by coordination and hydrogen bonds. Compound 1-purple shows reversible chromotropic behaviour in the solid state, changing from purple to dark blue (1-blue) upon heating or desorption. Interestingly, fast reversible blue to purple color transition can only be achieved in the presence of water or vapour. The chromism is demonstrated to stem from the quantitative desorption/adsorption of water by UV-vis-NIR spectroscopy and 2D IR correlation spectroscopy. In addition, the proton conductivity, water vapor adsorption and magnetic properties are also investigated. The dependence of coordinated water molecules with Co(II) centres on the proton conductivity is discussed.


 Please wait while we load your content...
Please wait while we load your content...