Synthesis of a bimetallic metal–organic framework catalyst via selective detection and adsorption of Fe3+ for enhanced bio-based catalysis†
Abstract
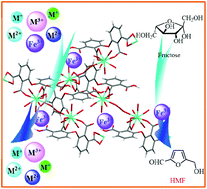
Bimetallic MOFs show a synergistic effect and enhanced properties compared to their monometallic counterparts; however, the topologies and functions of bimetallic MOFs are difficult to predict or control. Here, a 3D metal–organic framework (MOF), Tb-HODA, with an uncoordinated carboxyl group inner pore has been constructed, which exhibits good chemical resistance to both acidic and alkaline solutions with pH ranging from 2.5 to 12.5. Owing to the use of uncoordinated carboxyl groups within the channel as potential coordination sites for metal ions, this stable MOF exhibits a high sensitivity (KSV = 5.12 × 104 L mol−1) and a low detection limit (1.28 ppb) for sensing Fe3+ ions in aqueous solution, as well as a strong adsorption of Fe3+ with a potential to be a long-acting toxicide. Notably, the Lewis acidic property considerably increases when Tb-HODA adsorbs Fe3+ (Tb-HODA⊃Fe3+), which makes Tb-HODA⊃Fe3+ exhibit highly efficient and selective solid acid catalysis for the dehydration of fructose/glucose into 5-hydroxymethylfurfural.


 Please wait while we load your content...
Please wait while we load your content...