Pairing Suzuki–Miyaura cross-coupling and catalyst transfer polymerization
Abstract
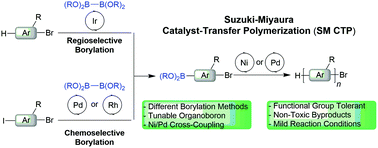
Suzuki–Miyaura catalyst transfer polymerization (SM CTP) is a versatile method to prepare conjugated polymers with control over molecular weight, sequence, and dispersity. This perspective aims to highlight some of the progress in using Suzuki–Miyaura coupling to prepare well-defined conjugated polymers via a chain-growth mechanism. We detail some of the advantages and challenges of this coupling to make aromatic polymers from monomers bearing two functional groups. The advances in arene borylation over the last twenty years are briefly highlighted, as these strategies should serve to diversify monomer scope in the future. The proposed mechanism for transmetalation in Suzuki–Miyaura polymerization is discussed, as it is different from the more typical Kumada coupling. We describe the versatility of the organoboron group used for this reaction and how it can be used tune polymerization behavior. Finally, some of the advances in catalyst design to prepare conjugated polymers using SM CTP are noted.
- This article is part of the themed collection: Polymer Chemistry Pioneering Investigators 2021


 Please wait while we load your content...
Please wait while we load your content...
