Asymmetric bismuth-rhodamines as an activatable fluorogenic photosensitizer†
Abstract
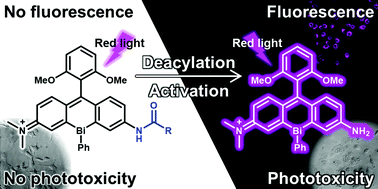
Bismuth–rhodamine compounds stand out for their unique excitable photosensitizing properties and concomitant fluorescence; however, further knowledge of the structure–property relationship is required to expand the scope of their practical application. With this aim, this study describes the first examples of asymmetric bismuth-incorporated rhodamines, BiRNH and BiRAc, including their synthesis, photophysical properties, and photosensitizing abilities. Upon red light excitation, BiRNH exhibits detectable emission and photosensitizing properties, while the N-acetylated derivative BiRAc shows a hypsochromic shift in the absorption wavelength and attenuation of emission and photosensitizing ability. These significantly different photophysical properties enabled us to design an activatable fluorogenic photosensitizer, BiRGlu, which bears a γ-glutamyl group instead of the acetyl group in BiRAc. The γ-glutamyl group can be cleaved by γ-glutamyl transpeptidase (GGT) to produce BiRNH, which acts as a red-light-excitable fluorophore and photosensitizer. A cell study revealed that the phototoxicity and fluorescence of BiRGlu could be simultaneously and selectively activated in the cells with high GGT activity. Thus, we established that BiRNH could be envisaged as a versatile scaffold for activatable fluorogenic photosensitizers.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...