Hydrogenolysis of glycerol in an aqueous medium over Pt/WO3/zirconium phosphate catalysts studied by 1H NMR spectroscopy†
Abstract
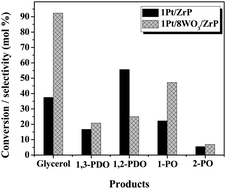
Bifunctional Pt/WO3/zirconium phosphate catalyzes the liquid-phase hydrogenolysis of glycerol in an aqueous medium. 1H NMR spectroscopy (solvent suppression pulse program) is employed to monitor this reaction. Propanediols (1,3 + 1,2-PDO) formed as the major product along with propanols (1- and 2-POs) as the minor product. A synergistic enhancement in glycerol conversion and selectivity to 1,3-PDO was observed when both Pt and WO3 were present in the catalyst. A volcano-shape variation of catalytic activity with W content was observed. A catalyst with 8 wt% W and 1 wt% Pt exhibited the highest selective hydrogenolysis performance (glycerol conversion = 92.3% and total PDOs selectivity = 45.9% and 1,3-PDO selectivity = 20.8% at 200 °C). Dispersed Pt in contact with polytungstate-type WO3 species was found to be the active catalytic site. 1H NMR spectroscopy is demonstrated as an attractive technique to quantify the products of a glycerol hydrogenolysis reaction.


 Please wait while we load your content...
Please wait while we load your content...