Small intestinal sampling capsule for inflammatory bowel disease type detection and management†
Abstract
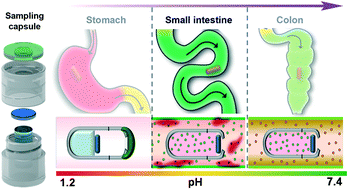
Although serum and fecal biomarkers (e.g., lactoferrin, and calprotectin) have been used in management and distinction between inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), none are proven to be a differential diagnostic tool between Crohn's disease (CD) and ulcerative colitis (UC). The main challenge with laboratory-based biomarkers in the stool test is the inability to indicate the location of the disease/inflammation in the gastrointestinal (GI) tract due to the homogenous nature of the collected fecal sample. For the first time, we have designed and developed a battery-free smart capsule that will allow targeted sampling of inflammatory biomarkers inside the gut lumen of the small intestine. The capsule is designed to provide a simple and non-invasive complementary tool to fecal biomarker analysis to differentiate the type of IBD by pinpointing the site of inflammatory biomarkers secretion (e.g., small or large bowel) throughout the GI tract. The capsule takes advantage of the rapid change from an acidic environment in the stomach to higher pH levels in the small intestine to dissolve a pH-sensitive polymeric coating as a means to activate the sampling process of the capsule within the small intestine. A swelling polyacrylamide hydrogel is placed inside the capsule as a milieu to collect the sampled GI fluid while also providing the required mechanical actuation to close the capsule once the sampling is completed. The hydrogel component along with the collected GI fluid can be easily obtained from the capsule through the screw-cap design for further extraction and analysis. As a proof of concept, the capsule's performance in sampling and extraction of bovine serum albumin (BSA) and calprotectin – a key biomarker of inflammation – was assessed within the physiologically relevant ranges. The ratio of extracted biomarkers relative to that in the initial sampling environment remained constant (∼3%) and independent of the sampling matrix in both in vitro and ex vivo studies. It is believed that the demonstrated technology will provide immediate impact in more effective IBD type differential diagnostic and treatment strategies by providing a non-invasive assessment of inflammation biomarkers profile throughout the digestive tract.
- This article is part of the themed collection: Lab on a Chip HOT Articles 2021


 Please wait while we load your content...
Please wait while we load your content...