Manganese oxide as an alternative to vanadium-based catalysts for effective conversion of glucose to formic acid in water†
Abstract
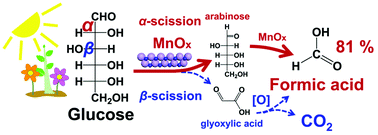
MnOx catalysts were synthesized under hydrothermal conditions for conversion of glucose into formic acid (FA) in water with the objective to develop vanadium-free reaction systems. An FA yield of 81.1% was obtained with a MnOx catalyst and glucose substrate in water at 160 °C and is almost twice the value obtained with vanadium-based heterogeneous catalysts. MnOx materials prepared hydrothermally at 100 °C had higher Mn2+/Mn3+ ratios and adsorbed oxygen species than those prepared at higher temperatures and gave the highest FA yields among the catalysts evaluated. Mechanistic studies of glucose–MnOx–water reaction systems revealed that two parallel reactions existed with arabinose being the intermediate in the α-scission route and glyoxylic acid being the intermediate in the β-scission route where CO2 co-forms with FA. Small water-soluble carbohydrates (glucose, sucrose, cellobiose, maltose, xylose) afforded FA yields greater than 50%, while starch afforded FA yields greater than 20%, thus demonstrating the potential of MnOx catalysts for converting biomass into FA.


 Please wait while we load your content...
Please wait while we load your content...