An ATP–Cu(ii) catalyst efficiently catalyzes enantioselective Michael reactions in water†
Abstract
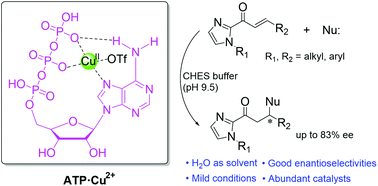
Biological hybrid catalysts have emerged as promising approaches to achieve enantioselective reactions in aqueous media. Enantioselective Michael reactions are one of the most important carbon–carbon bond formation reactions in organic synthesis, yet their aqueous-phase catalysis remains challenging. Herein, a single nucleotide-based catalyst (ATP·Cu2+) has been constructed using ATP and Cu2+ ions and catalyzes Michael reactions in aqueous media with high reactivities and good enantioselectivities up to 83% ee. The enantioselective catalytic performances of ATP·Cu2+ originate from specific binding between ATP and Cu2+ ions that exhibit both pH-dependent and temperature-dependent behaviors. The ATP·Cu2+ has been demonstrated to be successfully applied to a wide spectrum of enone substrates and carbon nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...