Visible-light-induced intermolecular aminoselenation of alkenes†
Abstract
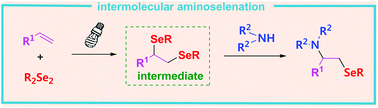
An intermolecular aminoselenation of alkenes with sulfonimides and diselenides is achieved via a visible-light-induced three component reaction. A broad variety of aminoselenation products is accessible in good yields with excellent functional group compatibility. Additional features of this new protocol include being additive- and photocatalyst-free and the use of natural sunlight as well as suitability for the modification of styrene-functionalized biomolecules. Mechanistic investigations suggest that the transformation occurs through a sequence of radical additions and nucleophilic substitutions.


 Please wait while we load your content...
Please wait while we load your content...